Protein-coding gene in the species Homo sapiens
| EZR |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
4RM8, 4RMA, 4RM9, 1NI2 |
|
|
| Identifiers |
|---|
| Aliases | EZR, CVIL, CVL, HEL-S-105, VIL2, Ezrin |
|---|
| External IDs | OMIM: 123900; MGI: 98931; HomoloGene: 55740; GeneCards: EZR; OMA:EZR - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 6 (human)[1] |
|---|
| | Band | 6q25.3 | Start | 158,765,741 bp[1] |
|---|
| End | 158,819,368 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 17 (mouse)[2] |
|---|
| | Band | 17|17 A1 | Start | 7,005,440 bp[2] |
|---|
| End | 7,050,183 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
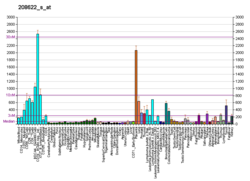
| Top expressed in | - ventricular zone
- bronchial epithelial cell
- ganglionic eminence
- nasal epithelium
- olfactory zone of nasal mucosa
- mucosa of paranasal sinus
- tendon of biceps brachii
- right adrenal cortex
- right uterine tube
- jejunal mucosa
|
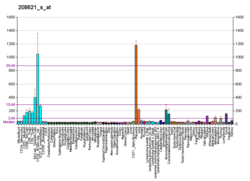
| | Top expressed in | - epithelium of stomach
- mucous cell of stomach
- left colon
- epithelium of small intestine
- intestinal villus
- ileum
- seminal vesicula
- Ileal epithelium
- pyloric antrum
- Epithelium of choroid plexus
|
| | More reference expression data |
|
|---|
| BioGPS | 
 | | More reference expression data |
|
|---|
|
|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Ezrin also known as cytovillin or villin-2 is a protein that in humans is encoded by the EZR gene.[5]
Structure
The N-terminus of ezrin contains a FERM domain which is further subdivided into three subdomains. The C-terminus contains an ERM domain.
Function
The cytoplasmic peripheral protein encoded by this gene can be phosphorylated by protein-tyrosine kinase in microvilli and is a member of the ERM protein family. This protein serves as a linker between plasma membrane and actin cytoskeleton. It plays a key role in cell surface structure adhesion, migration, and organization.[6]
The N-terminal domain (also called FERM domain) binds sodium-hydrogen exchanger regulatory factor (NHERF) protein (involving long-range allostery).[7] This binding can happen only when ezrin is in its active state. The activation of ezrin occurs in synergism of the two factors: 1) binding of the N-terminal domain to phosphatidylinositol(4,5)bis-phosphate (PIP2) and 2) phosphorylation of threonine T567 in the C-terminal domain.[8][9] Binding to actin filaments (via C-terminal) and to membrane proteins (via N-terminal) stabilizes the protein's conformation in its active mode. The membrane proteins like CD44 and ICAM-2 are indirect binding partners of ezrin, while EBP50 (ERM binding protein 50) can associate with ezrin directly.[10]
Interactions
VIL2 has been shown to interact with:
- CD43,[11]
- FASLG,[12][13]
- ICAM-1,[14]
- ICAM2,[14]
- ICAM3,[14][15]
- Merlin,[16]
- MSN,[12][17][18]
- PIK3R1,[19]
- PALLD[20]
- S100P,[21]
- SDC2,[22]
- SLC9A3R1,[23][24]
- SLC9A3R2,[25][26] and
- VCAM-1.[27]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000092820 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000052397 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Gould KL, Bretscher A, Esch FS, Hunter T (December 1989). "cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1". EMBO J. 8 (13): 4133–42. doi:10.1002/j.1460-2075.1989.tb08598.x. PMC 401598. PMID 2591371.
- ^ "Entrez Gene: VIL2 villin 2 (ezrin)".
- ^ Farago B, Li J, Cornilescu G, Callaway DJ, Bu Z (2010). "Activation of nanoscale allosteric protein domain motion revealed by neutron spin echo spectroscopy". Biophysical Journal. 99 (10): 3473–82. Bibcode:2010BpJ....99.3473F. doi:10.1016/j.bpj.2010.09.058. PMC 2980739. PMID 21081097.
- ^ Jayasundar JJ, Ju JH, He L, Liu D, Meilleur F, Zhao J, Callaway DJ, Bu Z (2012). "Open conformation of ezrin bound to phosphatidylinositol 4,5-bisphosphate and to F-actin revealed by neutron scattering". J. Biol. Chem. 287 (44): 37119–33. doi:10.1074/jbc.M112.380972. PMC 3481312. PMID 22927432.
- ^ Shabardina V, Kramer C, Gerdes B, Braunger J, Cordes A, Schaefer J, Mey I, Grill D, Gerke V, Steinem C (2016). "Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation". Biophys. J. 110 (12): 2710–2719. Bibcode:2016BpJ...110.2710S. doi:10.1016/j.bpj.2016.05.009. PMC 4919509. PMID 27332129.
- ^ Ivetic A, Ridley AJ (2004). "Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes". Immunology. 112 (2): 165–176. doi:10.1111/j.1365-2567.2004.01882.x. PMC 1782489. PMID 15147559.
- ^ Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, González-Amaro R, Sánchez-Mateos P, Sánchez-Madrid F (June 1998). "CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts". Blood. 91 (12): 4632–44. doi:10.1182/blood.V91.12.4632. PMID 9616160.
- ^ a b Gajate C, Mollinedo F (March 2005). "Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy". J. Biol. Chem. 280 (12): 11641–7. doi:10.1074/jbc.M411781200. PMID 15659383.
- ^ Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S (October 2000). "CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway". EMBO J. 19 (19): 5123–34. doi:10.1093/emboj/19.19.5123. PMC 302100. PMID 11013215.
- ^ a b c Heiska L, Alfthan K, Grönholm M, Vilja P, Vaheri A, Carpén O (August 1998). "Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate". J. Biol. Chem. 273 (34): 21893–900. doi:10.1074/jbc.273.34.21893. PMID 9705328.
- ^ Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sánchez-Madrid F (March 2002). "A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting". J. Biol. Chem. 277 (12): 10400–9. doi:10.1074/jbc.M110694200. PMID 11784723.
- ^ Grönholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpén O (March 1999). "Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin". J. Cell Sci. 112 (6): 895–904. doi:10.1242/jcs.112.6.895. PMID 10036239.
- ^ Gary R, Bretscher A (August 1995). "Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site". Mol. Biol. Cell. 6 (8): 1061–75. doi:10.1091/mbc.6.8.1061. PMC 301263. PMID 7579708.
- ^ Gary R, Bretscher A (November 1993). "Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins". Proc. Natl. Acad. Sci. U.S.A. 90 (22): 10846–50. Bibcode:1993PNAS...9010846G. doi:10.1073/pnas.90.22.10846. PMC 47875. PMID 8248180.
- ^ Gautreau A, Poullet P, Louvard D, Arpin M (June 1999). "Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway". Proc. Natl. Acad. Sci. U.S.A. 96 (13): 7300–5. Bibcode:1999PNAS...96.7300G. doi:10.1073/pnas.96.13.7300. PMC 22080. PMID 10377409.
- ^ Mykkänen OM, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, Carpén O (October 2001). "Characterization of human palladin, a microfilament-associated protein". Mol. Biol. Cell. 12 (10): 3060–73. doi:10.1091/mbc.12.10.3060. PMC 60155. PMID 11598191.
- ^ Koltzscher M, Neumann C, König S, Gerke V (June 2003). "Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P". Mol. Biol. Cell. 14 (6): 2372–84. doi:10.1091/mbc.E02-09-0553. PMC 194886. PMID 12808036.
- ^ Granés F, Urena JM, Rocamora N, Vilaró S (April 2000). "Ezrin links syndecan-2 to the cytoskeleton". J. Cell Sci. 113 (7): 1267–76. doi:10.1242/jcs.113.7.1267. PMID 10704377.
- ^ Brdicková N, Brdicka T, Andera L, Spicka J, Angelisová P, Milgram SL, Horejsí V (October 2001). "Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton". FEBS Lett. 507 (2): 133–6. doi:10.1016/s0014-5793(01)02955-6. PMID 11684085. S2CID 12676563.
- ^ Reczek D, Berryman M, Bretscher A (October 1997). "Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family". J. Cell Biol. 139 (1): 169–79. doi:10.1083/jcb.139.1.169. PMC 2139813. PMID 9314537.
- ^ Yun CH, Lamprecht G, Forster DV, Sidor A (October 1998). "NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin". J. Biol. Chem. 273 (40): 25856–63. doi:10.1074/jbc.273.40.25856. PMID 9748260.
- ^ Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL (September 2002). "The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation". J. Biol. Chem. 277 (36): 33188–95. doi:10.1074/jbc.M202522200. PMID 12080047.
- ^ Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (June 2002). "Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes". J. Cell Biol. 157 (7): 1233–45. doi:10.1083/jcb.200112126. PMC 2173557. PMID 12082081.
Further reading
- Martin TA, Harrison G, Mansel RE, Jiang WG (2004). "The role of the CD44/ezrin complex in cancer metastasis". Crit. Rev. Oncol. Hematol. 46 (2): 165–86. doi:10.1016/S1040-8428(02)00172-5. PMID 12711360.
 | This article on a gene on human chromosome 6 is a stub. You can help Wikipedia by expanding it. |
 1ni2: Structure of the active FERM domain of Ezrin
1ni2: Structure of the active FERM domain of Ezrin





















